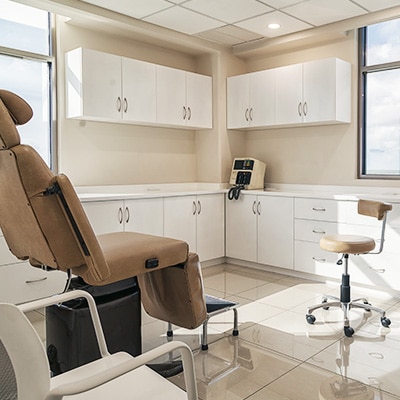
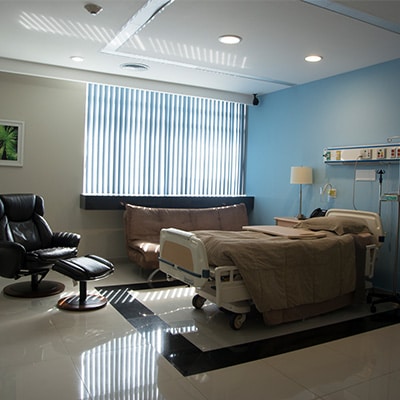
Why World Stem Cells Clinic?
Parents want better treatments for their children. They want a doctor who will listen to them without rolling his eyes. They want a kind of medical care where they get to ask questions and get sincere answers.
We have helped hundreds of these parents get their children closer to recovery. And we have a plan for you to do the same.
Quality Healthcare
We define quality of care as the total experience that our patients encounter from their very first contact with us to the follow up we provide long time after your visit. We see every interaction with our patients as an opportunity to share the newest science-based, medical research findings in the fields of stem cell therapies and autism. We believe that having an informed parent –in an effort to empower him and his family– is the only way to for us to deliver optimal healthcare.
Our Goal
is to provide:
Our Plan
Interview
We want to learn everything about your son or daughter. From you. Spend as much time as you (and our doctors) need in order to share everything about your child. From his family history, prenatal care, delivery, birth and development.
The treatment we are proposing will be fully explained by our doctors at this time and you will have as much time as you need to ask any question you might still have.
Treatment
Days 1 and 2 involve full medical checkup, planning and gathering of baseline labs.
Day 3 involves stem cells harvesting and reinfusions.
On day 4 there's additional stem cell reinfusions as well as an early stimulation session.
On day 5 all the medical information is explained, handed back to you and your child is discharged.
Follow Up
You are the hero of your child's journey.
Our therapy will help by healing some of the underlying gut and brain damage but it is everything that you do afterwards that will determine the extent of the improvement. That's why we continue working with you and your practitioner for a year after the therapy.
Our objective is to empower you to achieve the best result from it.
-

Dr Eduardo Ulloa, MD
Attending Physician
Dr Eduardo
-

Dr. Fredy Sansores, MD
Hematologist
Dr Fredy
-

Antonio Zamudio, CC
Lab Manager
Tony
-

Dr Rafael Gonzalez, PhD
Chief Scientific Officer
Dr Gonzalez
Built exclusively to process and store our patient's cells
Labs of this caliber are usually found inside large research hospitals. You know what else is found inside hospitals? Sick people. People with infectious diseases whose tissues end up in the same lab where other samples are being processed.
Our lab is only used for our patients. This makes our processing much safer since it is virtually impossible for our patient's samples to be contaminated by a sick person's pathogens.
Parents REALLY like that!
Meets the FDA's strict cGTP guidelines.
The cGTP requirements regulate the methods, the facilities and controls used for the manufacture of HCT/Ps (defined as: "articles containing or consisting of human cells or tissues that are intended for implantation, transplantation, infusion, or transfer into a human recipient.") in a way that prevents the introduction, transmission, or spread of communicable diseases by HCT/Ps (§ 1271.150(a)).